electronic configuration of he|Electron Configuration Calculator : Bacolod Hun 14, 2015 League 2 Betting Odds. View all available outright and match odds, plus get news, tips, free bets and money-back offers. All you need to bet.Hello guys I hope you all are doing well . Here is my another video . Hope you guys enjoy it . Be safe be happy . 😁•••••••••••DIRECT DOWNLOAD LINK MINI MILI.
electronic configuration of he,Mar 23, 2023 Electronic configuration [Rn] 5f 14 6d 10 7s 2 7p 6: Crystal structure (predicted) FCC .Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written . Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator.
Hun 14, 2015 Learning Objectives Derive the predicted ground-state electron configurations of atoms Identify and explain exceptions to predicted electron configurations for atoms and ions . Answer link. "He: " 1s^2 Helium, "He", has an atomic number equal to 2, which means that it has 2 protons in its nucleus. A neutral helium atom will thus have 2 electrons surrounding its nucleus. This means that the .The energy of atomic orbitals increases as the principal quantum number, n, increases. In any atom with two or more electrons, the repulsion between the.

The electron configurations of the elements are in Figure 6.9.2. Because each orbital can have a maximum of 2 electrons, there are 2 columns in the s block, 6 columns in the p block, 10 .
electronic configuration of he The electron configuration describes the position of electrons of an atom or a molecule in atomic or molecular orbitals. It is a type of code that shows the number of electrons in each atom’s energy level and how they are .Aluminum (atomic number 13), with 13 electrons and the electron configuration [Ne]3s 2 3p 1, is analogous to its family member boron, [He]2s 2 2p 1. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and .Therefore, the electronic configuration of sulfur can be written as 1s 2 2s 2 2p 6 3s 2 3p 4. The electronic configuration of elements can also be written with the help of noble gases. These noble gases have completely filled outermost .
The Aufbau Principle. We construct the periodic table by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli principle.We use the orbital energy diagram of Figure \(\PageIndex{1}\), recognizing that each .
electronic configuration of he Electron Configuration CalculatorThe Aufbau Principle. We construct the periodic table by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli principle.We use the orbital energy diagram of Figure \(\PageIndex{1}\), recognizing that each .Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, Subshell and Orbital by following certain rules. To Learn how to Write Electronic Configurations, Detailed Explanation, Filling of orbital with FAQs, Visit BYJU’S for detailed .Electron Configuration CalculatorHydrogen and helium are placed somewhat arbitrarily. Although hydrogen is not an alkali metal, its 1s 1 electron configuration suggests a similarity to lithium ([He]2s 1) and the other elements in the first column.Although helium, with a filled ns subshell, should be similar chemically to other elements with an ns 2 electron configuration, the closed principal shell dominates its . Similarity of valence shell electron configuration implies that we can determine the electron configuration of an atom solely by its position on the periodic table. Consider Se, as shown in Figure \(\PageIndex{10}\). It is in the fourth column of the p block. This means that its electron configuration should end in a p 4 electron

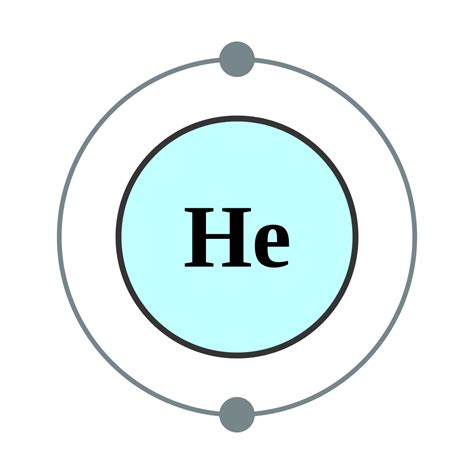
Aluminum (atomic number 13), with 13 electrons and the electron configuration [Ne]3s 2 3p 1, is analogous to its family member boron, [He]2s 2 2p 1. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron .Note that these electron configurations are given for neutral atoms in the gas phase, which are not the same as the electron configurations for the same atoms in chemical environments. In many cases, multiple configurations are within a small range of energies and the irregularities shown below do not necessarily have a clear relation to .The electronic configuration of the d-block elements in the advanced periodic table can be composed as displayed in the table beneath: 2 nd Series of Electronic Configuration. The electronic configuration of the d-block elements in the second series is as follows: 3 rd Series of Electronic ConfigurationElectron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H #1s^1# He . Helium, #"He"#, has an atomic number equal to #2#, which means that it has #2# protons in its nucleus.. A neutral helium atom will thus have #2# electrons surrounding its nucleus. This means that the electron .Figure \(\PageIndex{7}\): A core-abbreviated electron configuration (right) replaces the core electrons with the noble gas symbol whose configuration matches the core electron configuration of the other element. Similarly, the . Figure \(\PageIndex{1}\): Shell diagrams of hydrogen (H), helium (He), lithium (Li), and Berryellium (Be) atoms. (CC BY-SA 2.0 UK; Greg Robson modified by Pumbaa via Wikipedia) There are a set of general rules that are used to figure out the electron configuration of an atomic species: Aufbau Principle, Hund's Rule and the Pauli-Exclusion Principle. . Excited States of Helium. The lowest excitated state of helium is represented by the electron configuration 1s 2s.The 1s 2p configuration has higher energy, even though the 2s and 2p orbitals in hydrogen are degenerate, because the 2s penetrates closer to the nucleus, where the potential energy is more negative. When electrons are in different orbitals, their .Electron Configuration Chart for All Elements in the Periodic Table. There are 118 elements in the periodic table. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom.The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). For instance, the ground state electronic configuration of calcium (Z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from solving the .
electronic configuration of he|Electron Configuration Calculator
PH0 · What is the electron configuration for helium?
PH1 · List of Electron Configurations of Elements
PH2 · Helium Electron Configuration – Bohr and Aufbau Principle
PH3 · Electron Configuration for Helium (He)
PH4 · Electron Configuration Chart of All Elements (Full Chart)
PH5 · Electron Configuration Calculator
PH6 · Electron Configuration
PH7 · 6.9: Electron Configurations and the Periodic Table
PH8 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH9 · 3.4: Electronic Structure of Atoms (Electron Configurations)